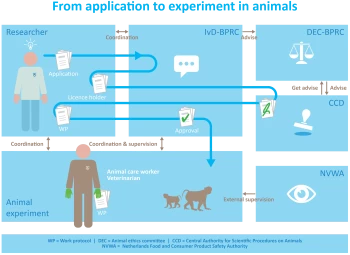
From application to experiment in animals
An experiment in animals is only allowed if there are no alternative methods. Prior to the actual experiment in animals, many steps must be taken. The research should be designed in such a way that we collect as much information as possible with as little discomfort as possible for the animals. Several authorities check if the discomfort expected outweighs the scientific interest. Here you can read how this process works.
Definitions & Abbreviations
The AWB-BPRC aims to ensure the optimal welfare of the laboratory animals in our institute. It is composed of our colony manager, two scientists, a veterinarian and a biotechnician.
The DEC-BPRC not only includes experts on animal research, alternatives for animal research, animal protection and welfare, and applied ethics and bioethics, but also non-experts from various sections of society. To ensure the committee's independence, the chairman of the committee as well as the majority of its members are not employed by BPRC. Because the review of research proposals involving primates requires specific expertise, the DEC-BPRC is often consulted by the CCD (see below) during the reviewing process. Of course, the CCD can also decide to consult another DEC.
This national authority issues a "project licence" to the licence holder.
This national body supervises animal welfare and the execution of animal experiments.

Project licences
The Animal Ethics Committee (DEC) is responsible for ethically evaluating whether the implications of the proposed animal experiment for the health and welfare of the animals outweigh the scientific and social interests.
An important part of this ethical evaluation is to thoroughly explore whether alternative methods can be used for the proposed animal experiment. If the intended answers can also be obtained
- without using laboratory animals (replacement),
- by using fewer laboratory animals (reduction)
- with a different experimental design in which the laboratory animals experience less discomfort (refinement),
the DEC will advise against the proposal. As a result, the proposed animal experiment will not be conducted.
On 18 December 2014, the amended Dutch Experiments on Animals Act (WoD) came into force. Because of this act, the process to obtain permission for conducting an animal experiment has changed considerably. New authorities have then been created, such as the Central Authority for Scientific Procedures on Animals (CCD) at a national level and the Animal Welfare Body (AWB) at an institutional level.

Important link
The AWB is an important link between the CCD and the researcher.
The mission of the AWB-BPRC is to aim for the best possible welfare of our laboratory animals in every aspect. This is done based on the principles of the 3Rs (Replacement, Reduction and Refinement), the intrinsic value of the animals and the current legal provisions. Furthermore, the AWB-BPRC supervises that the animal experiments are executed correctly within our institute.